Adaptação de lente escleral em doente após queratoplastia penetrante (PKP) com astigmatismo e anisometropia elevada
Managing a patient with scleral lens wear following Penetrating
Keratoplasty (PKP), high irregular astigmatism and large anisometropia
Ricardo Batista
Departamento das Ciências da Terapia e Reabilitação, Escola Superior de Tecnologia da Saúde de Lisboa, Instituto Politécnico
de Lisboa. Lisboa, Portugal. ricardo.batista@estesl.ipl.pt
ABSTRACT: Visual rehabilitation following penetrating keratoplasty (PKP) is the primary indi- cation for approximately 15% of all scleral lens fittings and significant irregular astigmatism is present in 62.9% of patients after this technique. Contact lenses (CL) can improve visual function in these patients, especially scleral lens (SL) since the lens is very stable and can vault the graft- -host interface, minimizing potential mechanical irritation from lens movement or bearing and reducing the potential for graft rejection or failure and correcting a high irregular corneal astig- matism. The other indication of SL is a monocular correction after PKP because of usually large anisometropia and aniseikonia induced. The combination of these two factors leads to success and indication to fit SL in these complex cases. This is a case report on a keratoconus patient suffe- ring from irregular astigmatism, large anisometropia, and generalized leukoma after a monocular PKP infection, with the main goals of improving best-corrected visual acuity (BCVA) and reduced aniseikonia with SL.
Keywords: Penetrating keratoplasty (PKP); Irregular astigmatism; Anisometropia; Scleral lens; Leukoma; Inflammation.
RESUMO: A reabilitação visual após queratoplastia penetrante (PKP) é a principal indicação para aproximadamente 15% de todas as adaptações de lentes esclerais e um astigmatismo irre- gular significativo está presente em 62,9% dos doentes após esta técnica. As lentes de contacto (LC) podem melhorar a função visual nestes doentes, especialmente as lentes esclerais (SL), uma vez que a lente é muito estável e pode ultrapassar a interface enxerto-hospedeiro, minimizando a potencial irritação mecânica relacionada com o movimento ou suporte da lente, reduzindo o potencial de rejeição ou falha do enxerto e corrigindo o astigmatismo corneano irregular elevado. Outra indicação da lente de contacto é a correção monocular após a PKP devido à anisometropia e aneisoconia normalmente presentes após esta técnica. A combinação destes dois fatores leva ao sucesso e à indicação da adaptação deste tipo de lentes de contacto nestes casos complexos. Este é um estudo de caso de um paciente com queratocone, que sofria de astigmatismo irregular, anisometropia elevada e leucoma generalizado após uma infeção monocular após PKP, com os principais objetivos de melhorar a acuidade visual corrigida (BCVA) e reduzir a aniseiconia com SL.
Palavras-chave: Queratoplastia penetrante (PKP); Astigmatismo irregular; Anisometropia; Lentes esclerais; Leucoma; Inflamação.
Introduction
Approximately 10% to 15% of patients diagnosed with
keratoconus require surgery and corneal transplantation is
the procedure employed6. The purpose of corneal transplantation for keratoconus is to replace the abnormal anterior refracting surface of the eye with a donor cornea that has a normal anterior surface shape. Corneal transplantation for keratoconus may be full-thickness (penetrating) or partial-thickness (lamellar)6. Various methods have been used to
manage post-PKP astigmatism, including suture djustment,selective suture removal, relaxing incisions, laser refractive surgery with customized laser ablation, laser in situ keratomileusis, and photorefractive keratectomy7. CL is used as the nonsurgical modality of choice for visual rehabilitation
after PKP1,3,7. Optical rehabilitation after penetrating keratoplasty (PKP) is the primary indication for approximately
15% of scleral lens fits1,3. Theoretically, SL is an ideal refractive correction for the post-graft eye because it can correct
highly irregular corneal astigmatism, which is very common
in these cases, and reduce any resultant anisometropia and
aniseikonia in unilateral cases3. SL is also extremely stable
compared with smaller-diameter corneal rigid lenses and, if
fitted appropriately, will vault the cornea entirely including
the graft-host junction, reducing the potential for mechanical irritation and tissue inflammation during lens wear1,3.
Despite this many indications, there is a major concern which
is corneal edema related to scleral lens wear because endothelial cell density decreases postoperatively, especially after more than 10 years, and will restrict the wearing time3-5.
This is a case report on a keratoconus patient suffering from
irregular astigmatism, large anisometropia, and generalized
leukoma after a monocular PKP infection, with the main goals
of improving best-corrected visual acuity (BCVA) and reduced
aniseikonia with SL.

Case report
A 63-year-old man presented with very low monocular visual acuity (OS), corrected with spectacles, and never used any kind of CL.
Ocular and medical history PKP for keratoconus in the left eye at the age of 48 years old followed by graft infection, with a history of dryness after the surgery and cataract extraction two years later of PKP.
Waiting for a regraft in the left eye but the surgeon would like to prolong the life of the current graft and proceed to a regraft only if absolutely necessary or/and tissue is available.
Keratoconus OD waiting for cataract extraction and later adapt CL too. The patient complains about intense photophobia and dysphotopsies, redness, and itching. Intraocular pressure (IOP) therapy with Azarga® (2x per day) and Xalatan® (1x per day). Spectacles correction OD: +1.00 –5.75 85° OS: -1.50. Visual acuity was 0.1 logMAR OD and ‘counting fingers’ at 1 meter on the left eye improved with pinhole to 1 logMAR.
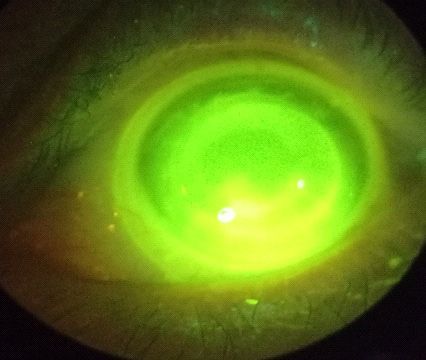
Slit-lamp examination
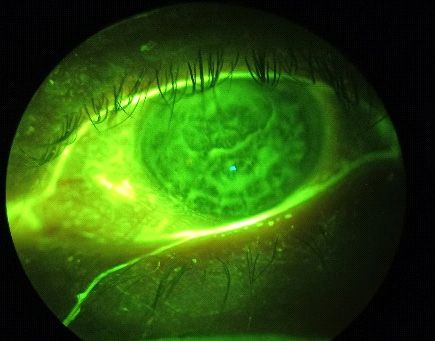
OS: Dry eye disease (cf. Figure 2), pseudophakia with his
posterior capsule IOL well centered and clear, 1+ (Efron Scale)
bulbar redness, significant scarring throughout the cornea,
stromal thickness, multiple corneal edema and haze around
the visual axis and prolate grafts, like we saw on Figure 1.
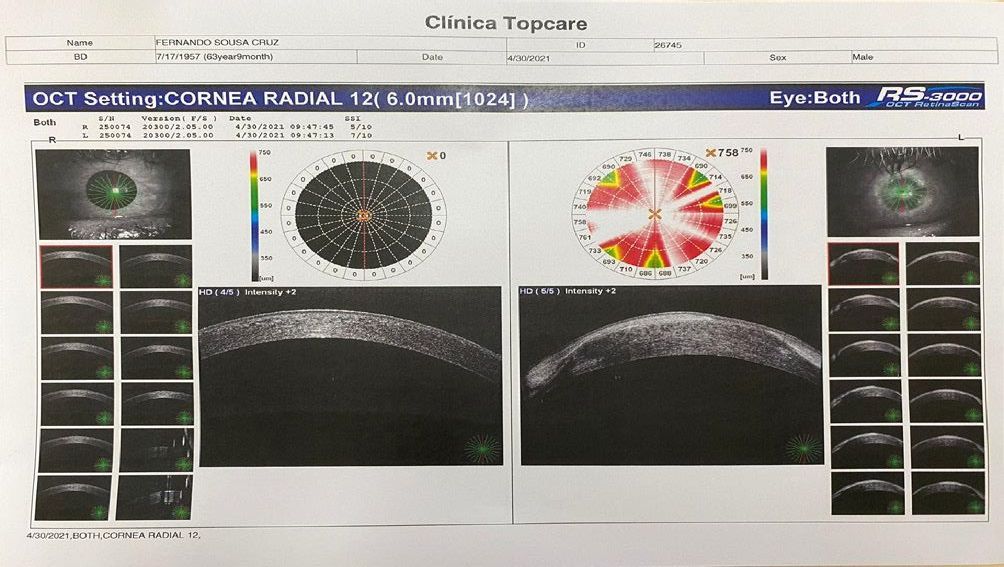
No topographic or specular microscopy acquisition OS
because of strong corneal edema. Only the OCT-SA examination was realized (cf. Figures 3 and 4), and we identify both in vertical and horizontal chord an hyperreflective scarring
areas and edema.
CL evaluation (08/April/2021)
Before CL fitting we measure IOP OS 16mmHg (iCare®) at
11 am.
Based on slit-lamp evaluation, OCT-SA, corneal condition,
and corneal diameter (CD) we start our SL fitting using trial
lenses from our fitting set ICD-FlexFit from Lenticon®.
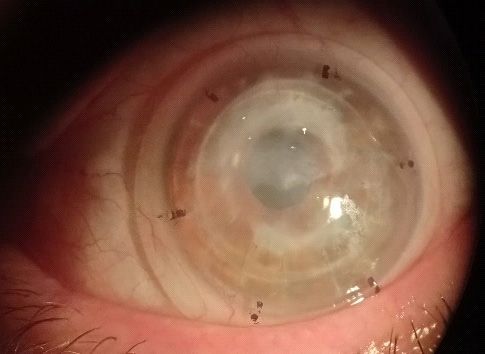
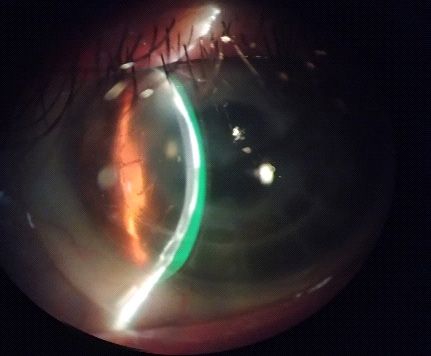
Initially, we started to determine overall lens diameter and limbal clearance based on CD measured (CD measures
11.5mm + 3.5mm = 15.00mm (Overall lens diameter) and
scribe markers (cf. Figure 4) using 14.80mm spherical lenses.
However, scribe markers were inside limbus and disaligned
inferiorly and temporarily (cf. Figure 5). Each scribe measures
0.60mm so it means that we have to increase the overall lens
diameter to 15.40mm.

CL evaluation (08/April/2021)
Before CL fitting we measure IOP OS 16mmHg (iCare®) at
11 am.
Based on slit-lamp evaluation, OCT-SA, corneal condition,
and corneal diameter (CD) we start our SL fitting using trial
lenses from our fitting set ICD-FlexFit from Lenticon®.
Initially, we started to determine overall lens diameter and limbal clearance based on CD measured (CD measures
11.5mm + 3.5mm = 15.00mm (Overall lens diameter) and
scribe markers (cf. Figure 4) using 14.80mm spherical lenses.
However, scribe markers were inside limbus and disaligned
inferiorly and temporarily (cf. Figure 5). Each scribe measures
0.60mm so it means that we have to increase the overall lens
diameter to 15.40mm.
Following the overall lens diameter determined and after
10 minutes of wear we evaluated the fluorogram, the trial lens
provided an initial central clearance of 200μm. The 200μm
central clearance was estimated as the amount of clearance
equal to the thickness of the lens (300μm). After a 60-minute
trial lens provided a central clearance of 100μm.
The next step was looking for a transition zone (TZ). In our
patient, we were in the presence of a 90° circumferentially
mid-peripheral touch and limbal bearing in the superiorly/
nasally quadrant so we increased TZR. The patient referred
discomfort precisely in that zone, not disappearing looking
left, right, up, and down and indicating an asymmetrical
shape of the sclera.
The trial lens’ peripheral haptics were evaluated as an
excessive edge lift in the superiorly/nasally quadrant.


Evaluation after 60 minutes.
Immediately after CL removal IOP value were 16.8mmHg
(iCare®) at 12 am.
Follow-up visit #1 (02/May/2021)
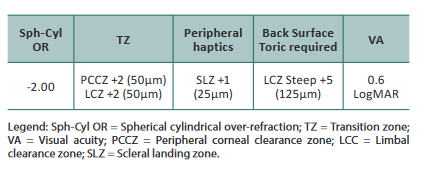
The patient returned three weeks later. The parameters of the lenses ordered are listed below.
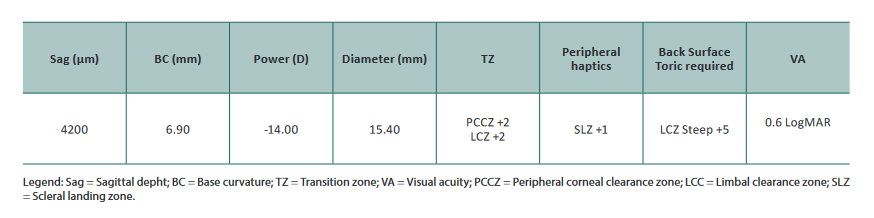
After 60min of wear, the eye displayed 350μm of central
apical clearance (Figure 7), PCCZ/LCZ touch at 180°
circumferentially superiorly (Figure 8), and good periph-
eral haptic zone but still disaligned inferior/temporally. The
patient still complained about discomfort in superiorly/
nasal, so we decided to increase the overall lens diameter to
16.30mm and back surface toricity to +8 (200μm).
With these changes in the new SL patient still complains
about discomfort in the same place so we decided to change
the geometry to an oblate.
Follow-up visit #2 (02/June/2021)
After the previous evaluation, we decide to change the geometry of SL to an oblate geometry and start using trial lenses from our fitting set Zenlens from Bausch&Lomb®.
Trial lens parameters are listed below
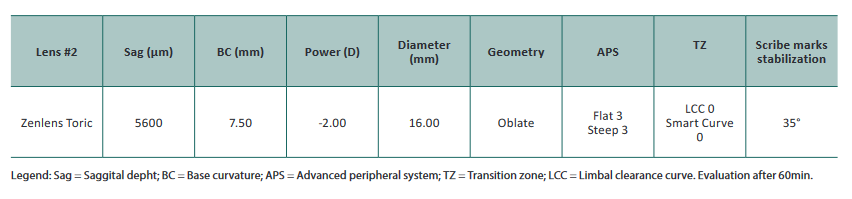
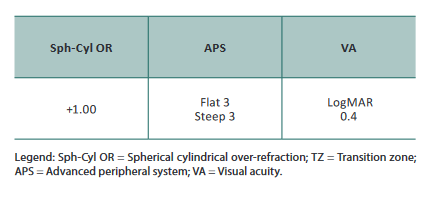
After 60min of wear, the eye displayed 300μm of central apical clearance, LCC vertically with slight touch in the superiorly/nasal quadrant (in corneal scarring zone) without discomfort but otherwise, excessive limbal clearance horizontally, good peripheral haptic zone but slightly disaligned inferior/temporally. After removal no corneal staining and edema.
After this evaluation, the only parameter that we changed was LCC with an increase vertically (+2) and a decrease horizontally (-2) and we reached the final SL for our patient.
Discussion
Penetrating keratoplasty has been performed as a treatment for keratoconus for over 70 years and involves the replacement of a full-thickness portion of the cornea despite the progress of surgical techniques.
Postoperative treatment continues to have numerous complications and patients may also not recover satisfactory VA after corneal surgery with post-keratoplasty regular or irregular astigmatism, and 10% to 20% of patients may have less than 20/200 after clear penetrating keratoplasty like present in our case6,10-11.
Many of them, such as graft rejection, significant astigmatism, cystoid macular edema, cataracts, and glaucoma lead to important limitations of the visual function11.
Glaucoma in particular, following PKP, has a relatively high frequency, it can appear early, as well as late in the evolution of the transplant, and is very important to recognize the most significant risk factors like preexisting glaucoma, lens status (i.e., aphakia, pseudophakia) and the disease for which PKP is performed. Once glaucoma has been installed after PKP, the medical treatment can control the IOP in certain cases and antiglaucomatous surgical interventions also contribute to the control of IOP11. Some studies suggest that SL has a minimal effect on IOP homeostasis in the normal eye during SL wear and an insignificant impact on the optic nerve head morphology in healthy adult eyes12 however still a large lack of studies in pathological eyes like in glaucoma that’s why is very important control IOP before and after SL wear. Cataract and glaucoma appear like a complication in this case report confirming the scientific evidence, that is why IOP measures are very important IOP measures before and after SL wear and only insert SL after 15m medication. In this case report, we are in line with the literature, observing a slight increase from 16.00mmHg to 16.8mmHg before and after removal respectively.
The cornea has a mechanism of protecting itself against bacterial, viral, or fungal infections but this mechanism often weakens after PKP procedures and could easily cause a graft infection that is what happened in our patient, resulting in significant scarring throughout the cornea, stromal thickness, multiple corneal edema, and haze around the visual axis and prolate grafts. Graft infection is known to occur after PKP and incidence varies from 1.76% to 12.1%9. Pre-existing graft failure, extended interval between donor death and PKP, and fungal infection were important risk factors for treatment failure of graft infection5,9.
Another important issue is to evaluate the relationship between donor age and graft survival after PKP on long-term endothelial cell loss (using specular microscopy). Substantial cell loss occurs in eyes with a clear graft 10 years after PKP, with the rate of cell loss being slightly greater with older donor age and greater preoperative endothelial cell density5. Our patient has a graft of 15 years, although we do not know the donor’s age and was impossible to measure endothelial cell density, but is expected to have a very low endothelial cell density with pleomorphism and polimegathism which presents a big limitation with SL. The authors instructed their patients to remove their SL every six hours to increase oxygen delivery to the cornea and remove the stagnant post-lens fluid reservoir, which may have contributed to their relatively low failure rate and that is the strategy used with our patient3.
CL is used as the nonsurgical modality of choice for visual rehabilitation after PKP1,3,7. Optical rehabilitation after PKP is the primary indication for approximately 15% of scleral lens fits1,3. Theoretically, SL is an ideal refractive correction for the post-graft eye because it can correct highly irregular corneal astigmatism, which is very common in these cases, and reduce any resultant anisometropia and aniseikonia in unilateral cases1,3. We can improve BCVA from counting fingers at 1 meter to 0,6 LogMAR, presenting a significant increase.
When fitting a post-graft patient with a CL, there are multiple factors that must be considered. These include the diameter of the graft, the topographical relationship between the host and donor cornea, the toricity present in the graft, and the location of the graft1. In our patient with first CL order observed a TZ touch at 180° circumferentially superiorly, which is why we increased the overall diameter and created more oblate geometry. When a corneal graft is flatter than the host cornea, the graft is termed ‘proud’ or ‘sunken’2.
Another limitation of these cases was the excessive limbal clearance which could cause conjunctival prolapse, however, we must increase the overall diameter, create more oblate geometry, and then reduce LCC to avoid excessive limbal clearance and limbal stem cell compression. Our patient had only an excessive limbal clearance at the horizontal meridian, which is why only decreased horizontal LCC (-2). Limbal stem cells play a crucial role in replacing epithelial cells and acting as a barrier between the avascular cornea and vascularized conjunctival tissue. If limbal stem cells are compromised, corneal reepithelization by the conjunctiva can cause opacification of the cornea and poor vision1-2.
Despite all these limitations, the main problem still remains corneal edema. Post-penetrating keratoplasty eyes fitted with scleral lenses exhibit more corneal edema (2.99%) and greater variability in the corneal response compared with healthy eyes after a short period of lens wear. Further longer-term studies are required to determine corneal characteristics of post-penetrating keratoplasty eyes that may potentially contraindicate scleral lens wear. While these studies do not provide us with some guidelines, we suggest reducing wearing time or incorporating a period of lens removal throughout the day. Fenestrated scleral lenses may also be a viable option when fitting post-penetrating keratoplasty eyes because of their lower corneal clearance, altered tear exchange, and reduced suction forces1,3,5,9.
Scleral lens despite all limitations presents as a viable option in post-graft patients, especially in monocular cases correcting large anisometropia and aniseikonia and improving BCVA. We have been able to wear SL for at least six hours and avoid the need for a regraft and the possible negative outcomes associated with the surgical procedure12.
Recently, we had available in Portugal a profilometer that would allow us a better knowledge of the topographic profile at the peripheral cornea, limbus, and anterior sclera and, consequently, provide a consistent basis for more optimized designs of scleral contact lenses, better comfort and fitting in extremely difficult cases like this one. However, at the beginning of the fit process, it was not yet available.
In summary, patients with this type of diagnosis are always quite challenging. A rigorous pre-fit evaluation is required with the study of corneal endothelial status (specular microscopy), IOP, corneal topography (oblate/prolate donor cornea and limbus), scleral topography (APS of SL) and slit lamp (edema, corneal scarring, etc.). With the SL fitted, avoid central and limbal excessive vault, good scleral alignment, use SL material with the highest Dk available, control the wearing time (not exceed 6h per/day), measure IOP immediately after removal of SL and in regular check-up visits and make regular visits to control, especially edema and neovascularization.
References
1. Kumar M, Shetty R, Lalgudi VG, Vincent SJ. Scleral lens wear following penetrating keratoplasty: changes in corneal curvature and optics. Ophthalmic Physiol Opt. 2020;40(4):502-9.
2. Wietharn BE, Driebe Jr WT. Fitting contact lenses for visual rehabilitation after penetrating keratoplasty. Eye Contact Lens. 2004;30(1):31-3.
3. Kumar M, Shetty R, Khamar P, Vincent SJ. Scleral lens-induced corneal edema after penetrating keratoplasty. Optom Vis Sci. 2020;97(9):697-702.
4. Culbertson WW, Abbott RL, Forster RK. Endothelial cell loss in penetrating keratoplasty. Ophthalmology. 1982;89(6):600-4.
5. Lass JH, Benetz BA, Gal RL, Kollman C, Raghinaru D, Dontchev M, et al. Donor age and factors related to endothelial cell loss 10 years after penetrating keratoplasty: Specular Microscopy Ancillary study. Ophthalmology. 2013;120(12):2428-35.
6. Javadi MA, Feizi S, Yazdani S, Mirbabaee F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: a clinical trial. Cornea. 2010;29(4):365-71.
7. Barnett M, Lien V, Li JY, Durbin-Johnson B, Mannis MJ. Use of scleral lenses and miniscleral lenses after penetrating keratoplasty. Eye Contact Lens. 2016;42(3):185-9.
8. Fujita A, Yoshida J, Toyono T, Usui T, Miyai T. Severity assessment of acute hydrops due to recurrent keratoconus after penetrating keratoplasty using anterior segment optical coherence tomography. Curr Eye Res. 2019;44(11):1189-94.
9. Sung MS, Choi W, You IC, Yoon KC. Factors affecting treatment outcome of graft infection following penetrating keratoplasty. Korean J Ophthalmol. 2015;29(5):301-8.
10. Suarez C, Madariaga V, Lepage B, Malecaze M, Fournié P, Soler V, et al. First experience with the ICD 16.5 mini-scleral lens for optic and therapeutic purposes. Eye Contact Lens. 2018;44(1):44-9.
11. Zemba M, Stamate AC. Glaucoma after penetrating keratoplasty. Rom J Ophthalmol. 2017;61(3):159-65.
12. Walker MK, Pardon LP, Redfern R, Patel N. IOP and optic nerve head morphology during scleral lens wear. Optom Vis Sci. 2020;97(9):661-8.
Conflito de interesses
O autor declara não possuir quaisquer conflitos de interesse.
Artigo submetido em 11.11.2021 e aprovado em 23.06.2023





